OUR RESEARCH
The NeuroPalm Lab is a molecular neuroscience lab interested in how neurons use the protein-lipid modification palmitoylation to target proteins to specific locations and to define how that contributes to normal brain function and how it goes wrong in brain diseases. Neurons are the basic working unit of the brain. They transmit information from other neurons or sensory organs to downstream neurons or muscles and, in doing so, control learning and memory, movement, and other complex tasks. Protein localization is critical for brain function but can be challenging in large, complex neurons.
WHAT IS PALMITOYLATION?
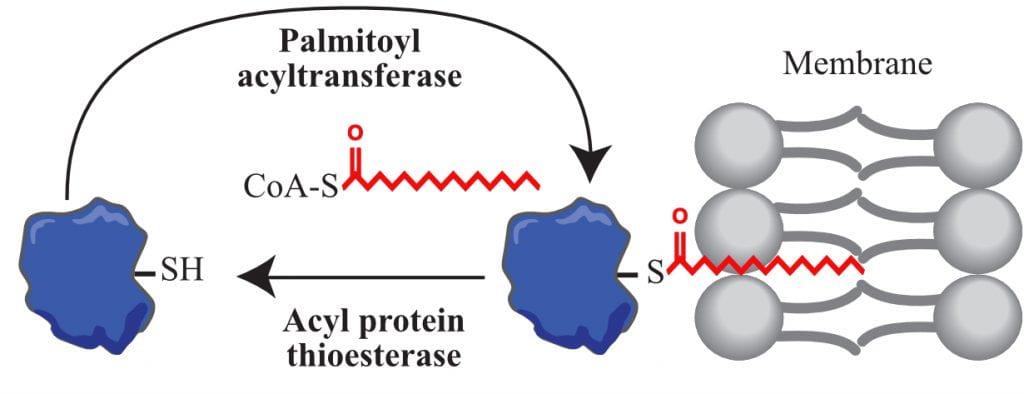
Palmitoylation acts like a sticky tag to direct and tether proteins to specific locations within the cell and is the addition of long chain fatty acids, commonly palmitate, to cysteine residues of proteins. Palmitoylation can be added or removed to proteins by enzymes called palmitoyl acyltransferases and acyl protein thioesterases, respectively.
CURRENT RESEARCH QUESTIONS
Precise control of neuronal excitability, or whether or not a neuron fires a nerve impulse to its neighbours, is essential for normal behaviour and cognition, while aberrant excitability is a hallmark of many neurological diseases, including epilepsy, bipolar disorder, Schizophrenia, and episodic ataxias. A key factor that controls the threshold of excitability is the clustering of ion channels and receptors at sites of excitability, including synapses, the axon initial segment (AIS), and nodes of Ranvier; but how such clustering is regulated is not fully understood. Current areas of research in the NeuroPalm lab include:
- How does palmitoylation control voltage-gated sodium and potassium channel targeting and function at the AIS and nodes of Ranvier?
- How do physiological and pathological changes in neuronal activity impact potassium and sodium channel palmitoylation and targeting to the AIS?
- What palmitoyl acyltransferase and palmitoyl protein thioesterase enzymes palmitoylate and depalmitoylate, respectively, sodium and potassium ion channels and how are they regulated by physiological and pathological changes in neuronal activity?
- Do human mutations in ion channels that cause ataxia and seizure disorders alter channel palmitoylation and targeting to sites of excitability?
- How does palmitoylation regulate axonal trafficking of cargo to sites of neuronal excitability?
We use cutting edge genetic, biochemical, and cell biological approaches to answer these questions, including CRISPR-mediated gene knockout and mutation, shRNA-mediated knockdown and rescue, specialized palmitoylation assays, and live and fixed confocal microscopy in neuronal cultures grown in conventional culture and in microfluidic devices.